What Causes Molecules To Absorb Uv And Visible Light . Explains what is happening when organic molecules absorb uv or visible light, and why it varies from compound to compound Here, we see that the extended system of conjugated pi bonds causes the molecule to absorb light in the visible range. This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light. The absorbed photon excites an. Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. A black light looks dark purple, but most of the light it emits is in the ultraviolet (uv) range.
from www3.nd.edu
The absorbed photon excites an. Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light. Explains what is happening when organic molecules absorb uv or visible light, and why it varies from compound to compound A black light looks dark purple, but most of the light it emits is in the ultraviolet (uv) range. Here, we see that the extended system of conjugated pi bonds causes the molecule to absorb light in the visible range.
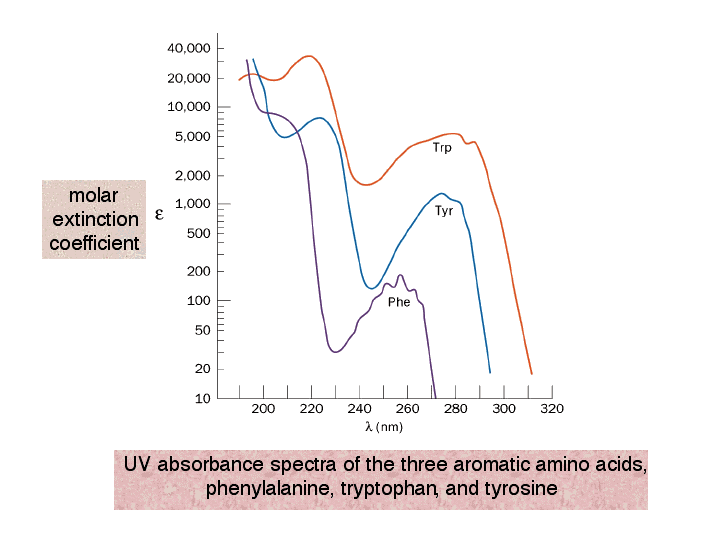
UV absorbance spectra of the three aromatic amino acids, phenylalanine
What Causes Molecules To Absorb Uv And Visible Light Explains what is happening when organic molecules absorb uv or visible light, and why it varies from compound to compound Explains what is happening when organic molecules absorb uv or visible light, and why it varies from compound to compound A black light looks dark purple, but most of the light it emits is in the ultraviolet (uv) range. This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light. The absorbed photon excites an. Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. Here, we see that the extended system of conjugated pi bonds causes the molecule to absorb light in the visible range.
From www.mdpi.com
Molecules Free FullText Ultraviolet Radiation, Aging and the Skin What Causes Molecules To Absorb Uv And Visible Light Explains what is happening when organic molecules absorb uv or visible light, and why it varies from compound to compound Here, we see that the extended system of conjugated pi bonds causes the molecule to absorb light in the visible range. The absorbed photon excites an. This page explains what happens when organic compounds absorb uv or visible light, and. What Causes Molecules To Absorb Uv And Visible Light.
From bitesizebio.com
How Fluorescent Molecules Work What Causes Molecules To Absorb Uv And Visible Light This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light. Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. Explains what is happening when organic molecules absorb uv or visible light, and why it varies from compound to compound Here, we see that the. What Causes Molecules To Absorb Uv And Visible Light.
From chem.libretexts.org
4 Detection and Absorption of Ultraviolet Light (Experiment What Causes Molecules To Absorb Uv And Visible Light Here, we see that the extended system of conjugated pi bonds causes the molecule to absorb light in the visible range. This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light. Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. Explains what is happening. What Causes Molecules To Absorb Uv And Visible Light.
From ivypanda.com
What Causes Molecules to Absorb Light 631 Words Report Example What Causes Molecules To Absorb Uv And Visible Light Explains what is happening when organic molecules absorb uv or visible light, and why it varies from compound to compound Here, we see that the extended system of conjugated pi bonds causes the molecule to absorb light in the visible range. A black light looks dark purple, but most of the light it emits is in the ultraviolet (uv) range.. What Causes Molecules To Absorb Uv And Visible Light.
From www3.nd.edu
UV absorbance spectra of the three aromatic amino acids, phenylalanine What Causes Molecules To Absorb Uv And Visible Light The absorbed photon excites an. Explains what is happening when organic molecules absorb uv or visible light, and why it varies from compound to compound A black light looks dark purple, but most of the light it emits is in the ultraviolet (uv) range. Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores.. What Causes Molecules To Absorb Uv And Visible Light.
From www.youtube.com
A.8.3 State that organic molecules containing a double bond absorb UV What Causes Molecules To Absorb Uv And Visible Light Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. Here, we see that the extended system of conjugated pi bonds causes the molecule to absorb light in the visible range. A black light looks dark purple, but most of the light it emits is in the ultraviolet (uv) range. Explains what is happening. What Causes Molecules To Absorb Uv And Visible Light.
From www.youtube.com
Which molecules absorb UVVis light? YouTube What Causes Molecules To Absorb Uv And Visible Light This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light. Explains what is happening when organic molecules absorb uv or visible light, and why it varies from compound to compound Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. A black light looks dark. What Causes Molecules To Absorb Uv And Visible Light.
From www.masterorganicchemistry.com
What is UVVis Spectroscopy? And How Does It Apply To Conjugation? What Causes Molecules To Absorb Uv And Visible Light This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light. The absorbed photon excites an. A black light looks dark purple, but most of the light it emits is in the ultraviolet (uv) range. Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. Here,. What Causes Molecules To Absorb Uv And Visible Light.
From www.masterorganicchemistry.com
What is UVVis Spectroscopy? And How Does It Apply To Conjugation? What Causes Molecules To Absorb Uv And Visible Light Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light. The absorbed photon excites an. A black light looks dark purple, but most of the light it emits is in the ultraviolet (uv) range. Here,. What Causes Molecules To Absorb Uv And Visible Light.
From www.researchgate.net
Spectrum of ultraviolet (UV) light and wavelengthdependent What Causes Molecules To Absorb Uv And Visible Light Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. The absorbed photon excites an. This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light. Here, we see that the extended system of conjugated pi bonds causes the molecule to absorb light in the visible. What Causes Molecules To Absorb Uv And Visible Light.
From www.researchgate.net
Principle of UVVIS spectrophotometry. (a) Detected UVVIS spectrum for What Causes Molecules To Absorb Uv And Visible Light The absorbed photon excites an. This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light. Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. Explains what is happening when organic molecules absorb uv or visible light, and why it varies from compound to compound. What Causes Molecules To Absorb Uv And Visible Light.
From ar.inspiredpencil.com
Wavelength Of Light Photosynthesis What Causes Molecules To Absorb Uv And Visible Light The absorbed photon excites an. This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light. A black light looks dark purple, but most of the light it emits is in the ultraviolet (uv) range. Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. Explains. What Causes Molecules To Absorb Uv And Visible Light.
From socratic.org
How does ozone protect us? Socratic What Causes Molecules To Absorb Uv And Visible Light Explains what is happening when organic molecules absorb uv or visible light, and why it varies from compound to compound Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. A black light looks dark purple, but most of the light it emits is in the ultraviolet (uv) range. The absorbed photon excites an.. What Causes Molecules To Absorb Uv And Visible Light.
From courses.lumenlearning.com
Infrared Spectroscopy MCC Organic Chemistry What Causes Molecules To Absorb Uv And Visible Light This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light. The absorbed photon excites an. Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. Here, we see that the extended system of conjugated pi bonds causes the molecule to absorb light in the visible. What Causes Molecules To Absorb Uv And Visible Light.
From onlinelibrary.wiley.com
Ultraviolet radiation oxidative stress affects eye health Ivanov What Causes Molecules To Absorb Uv And Visible Light Here, we see that the extended system of conjugated pi bonds causes the molecule to absorb light in the visible range. Explains what is happening when organic molecules absorb uv or visible light, and why it varies from compound to compound This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light.. What Causes Molecules To Absorb Uv And Visible Light.
From www.promegaconnections.com
NGS quantitation Archives Promega Connections What Causes Molecules To Absorb Uv And Visible Light This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light. Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. A black light looks dark purple, but most of the light it emits is in the ultraviolet (uv) range. The absorbed photon excites an. Explains. What Causes Molecules To Absorb Uv And Visible Light.
From www.vision-doctor.com
UV illumination What Causes Molecules To Absorb Uv And Visible Light Here, we see that the extended system of conjugated pi bonds causes the molecule to absorb light in the visible range. Explains what is happening when organic molecules absorb uv or visible light, and why it varies from compound to compound The absorbed photon excites an. Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they. What Causes Molecules To Absorb Uv And Visible Light.
From chem.libretexts.org
4.5 Ultraviolet and visible spectroscopy Chemistry LibreTexts What Causes Molecules To Absorb Uv And Visible Light Most molecules and ions absorb energy in the ultraviolet or visible range, i.e., they are chromophores. The absorbed photon excites an. A black light looks dark purple, but most of the light it emits is in the ultraviolet (uv) range. This page explains what happens when organic compounds absorb uv or visible light, and why the wavelength of light. Here,. What Causes Molecules To Absorb Uv And Visible Light.